Burns, JE;
Carlin, FE;
(2020)
Experience of conducting clinical trials of investigational medicinal products during a respiratory virus pandemic: Lessons learnt from COVID-19.
Clinical Trials
10.1177/1740774520974113.
(In press).

Preview |
Text (Article)
Clinical Trials_covid_trial_experiences_1.2_final_accepted.pdf - Accepted Version Download (619kB) | Preview |
![[thumbnail of Figure 1]](https://discovery.ucl.ac.uk/10117609/10.hassmallThumbnailVersion/Figure_1.jpg) 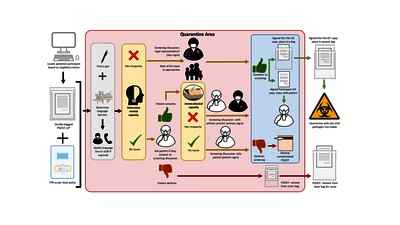 Preview |
Image (Figure 1)
Figure_1.jpg - Accepted Version Download (635kB) | Preview |
| Type: | Article |
|---|---|
| Title: | Experience of conducting clinical trials of investigational medicinal products during a respiratory virus pandemic: Lessons learnt from COVID-19 |
| Location: | England |
| Open access status: | An open access version is available from UCL Discovery |
| DOI: | 10.1177/1740774520974113 |
| Publisher version: | http://dx.doi.org/10.1177/1740774520974113 |
| Language: | English |
| Additional information: | This version is the author accepted manuscript. For information on re-use, please refer to the publisher’s terms and conditions. |
| UCL classification: | UCL UCL > Provost and Vice Provost Offices > School of Life and Medical Sciences UCL > Provost and Vice Provost Offices > School of Life and Medical Sciences > Faculty of Population Health Sciences UCL > Provost and Vice Provost Offices > School of Life and Medical Sciences > Faculty of Population Health Sciences > Institute for Global Health |
| URI: | https://discovery.ucl.ac.uk/id/eprint/10117609 |
Downloads since deposit
217Downloads
Download activity - last month
Download activity - last 12 months
Downloads by country - last 12 months
Archive Staff Only
 |
View Item |


