Vormittag, P;
Gunn, R;
Ghorashian, S;
Veraitch, FS;
(2018)
A guide to manufacturing CAR T cell therapies.
Current Opinion in Biotechnology
, 53
pp. 164-181.
10.1016/j.copbio.2018.01.025.

Preview |
Text (Article)
Veraitch_20180109_CAR T cell manufacturing Review - Revision.pdf - Accepted Version Download (1MB) | Preview |
Preview |
Text (Highlights)
Veraitch_CAR T cell manufacturing review - Highlights.pdf - Accepted Version Download (102kB) | Preview |
![[thumbnail of Graphical Abstract]](https://discovery.ucl.ac.uk/10050033/13.hassmallThumbnailVersion/Veraitch_Graphical%20Abstract.png) 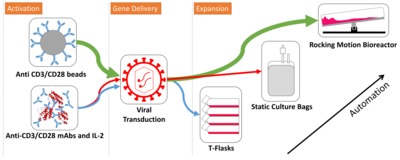 Preview |
Image (Graphical Abstract)
Veraitch_Graphical Abstract.png - Accepted Version Download (2MB) | Preview |
![[thumbnail of Figure 1]](https://discovery.ucl.ac.uk/10050033/18.hassmallThumbnailVersion/Veraitch_Figure%201.png) 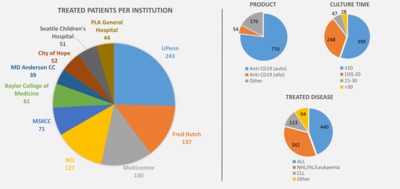 Preview |
Image (Figure 1)
Veraitch_Figure 1.png - Accepted Version Download (1MB) | Preview |
![[thumbnail of Figure 2]](https://discovery.ucl.ac.uk/10050033/23.hassmallThumbnailVersion/Veraitch_Figure%202.png) 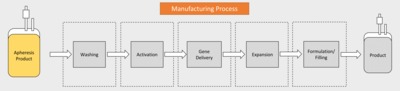 Preview |
Image (Figure 2)
Veraitch_Figure 2.png - Accepted Version Download (1MB) | Preview |
![[thumbnail of Figure 3]](https://discovery.ucl.ac.uk/10050033/28.hassmallThumbnailVersion/Veraitch_Figure%203.png) 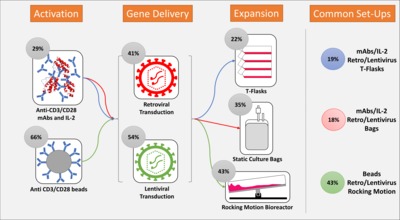 Preview |
Image (Figure 3)
Veraitch_Figure 3.png - Accepted Version Download (2MB) | Preview |
Abstract
In recent years, chimeric antigen receptor (CAR) modified T cells have been used as a treatment for haematological malignancies in several phase I and II trials and with Kymriah of Novartis and Yescarta of KITE Pharma, the first CAR T cell therapy products have been approved. Promising clinical outcomes have yet been tempered by the fact that many therapies may be prohibitively expensive to manufacture. The process is not yet defined, far from being standardised and often requires extensive manual handling steps. For academia, big pharma and contract manufacturers it is difficult to obtain an overview over the process strategies and their respective advantages and disadvantages. This review details current production processes being used for CAR T cells with a particular focus on efficacy, reproducibility, manufacturing costs and release testing. By undertaking a systematic analysis of the manufacture of CAR T cells from reported clinical trial data to date, we have been able to quantify recent trends and track the uptake of new process technology. Delivering new processing options will be key to the success of the CAR-T cells ensuring that excessive manufacturing costs do not disrupt the delivery of exciting new therapies to the wide possible patient cohort.
| Type: | Article |
|---|---|
| Title: | A guide to manufacturing CAR T cell therapies |
| Location: | England |
| Open access status: | An open access version is available from UCL Discovery |
| DOI: | 10.1016/j.copbio.2018.01.025 |
| Publisher version: | http://dx.doi.org/10.1016/j.copbio.2018.01.025 |
| Language: | English |
| Additional information: | This version is the author accepted manuscript. For information on re-use, please refer to the publisher’s terms and conditions. |
| UCL classification: | UCL UCL > Provost and Vice Provost Offices > School of Life and Medical Sciences UCL > Provost and Vice Provost Offices > School of Life and Medical Sciences > Faculty of Population Health Sciences > UCL GOS Institute of Child Health UCL > Provost and Vice Provost Offices > UCL BEAMS UCL > Provost and Vice Provost Offices > UCL BEAMS > Faculty of Engineering Science UCL > Provost and Vice Provost Offices > UCL BEAMS > Faculty of Engineering Science > Dept of Biochemical Engineering |
| URI: | https://discovery.ucl.ac.uk/id/eprint/10050033 |
Archive Staff Only
 |
View Item |


